Fertility neurobiology, or how stress influences fertilization difficulties


It is well known that long-term stress leads to many disorders, such as depression, anxiety disorders, cardiovascular diseases, weakened immunity, and reduced fertility. However, it is also known that we are different from each other. Some of us are more resistant to stress and others are more sensitive. The same events in one person will lead to reduced fertility or other problems, and in another it will not. What does it depend on and how do the hormones and neurotransmitters in our brain affect fertility?
To answer this question, Cynthia Bethea, Maria Centeno and Judy Cameron conducted in 2008 a study on the effects of stress on fertility in monkeys. 15 female macaques were subjected to various types of stress for several to dozen of weeks. In order to induce psychosocial stress, they were moved to a new room and placed with individuals they did not know before, moreover their food rations were reduced by 20% and they performed intensive physical exercise for an hour 5 days a week. All these factors – both mental stress and malnutrition as well as intense exercise – reduce fertility also in humans.
The experiment showed that although all monkeys had the same conditions, one third of them experienced menstrual cycle disorders and ovulation very quickly, while the other third did not notice any negative changes over a dozen or so weeks. There was also a group with moderate stress sensitivity who developed ovulation disorders, but only after a few weeks.
They found that the differences in the effects of stress on fertility in these animals were due to differences in the levels of hormones and neurotransmitters in their brains. Even in the period when they were not subjected to stressful conditions, the monkeys differed in the level of e.g. serotonin, gamma-aminobutyric acid, corticoliberin and proopiomelanocortin.
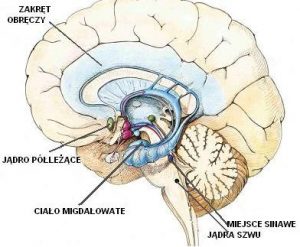
Serotonin
Serotonin plays a key role in regulating mood and emotions, cognitive functions (such as learning, memory), regulation of the satiety center, and numerous functions of the autonomic nervous system. Reduced activity of the serotonin system occurs in people with increased sensitivity to stress and anxiety disorders, depression, alcoholism, anorexia, as well as in people suffering from PMS.
Lower endogenous serotonin levels make a person more sensitive to stress, while stress itself also reduces serotonin levels. Serotonin levels are also related to ovarian steroid hormones (e.g. estradiol, progesterone). Reduced levels of these hormones lead to a reduction in serotonin levels.
Research by Bethea and colleagues showed that stress-sensitive monkeys, i.e. those with a rapid onset of fertility impairment, had fewer serotonin neurons, lower expression of genes related to this neurotransmitter in the dorsal part of the raphe nucleus (the area of the brainstem with nerve cells that release serotonin into the brain), as well as lower levels of estradiol and progesterone than stress-tolerant monkeys.
Serotonin receptors in the hypothalamus and the GABA neurotransmitter
The serotonin receptors in the hypothalamus and the GABA neurotransmitter (gamma-aminobutyric acid) are also important for fertility and stress response. There are several types of serotonin receptors. In the hypothalamus there are 5HT1A, 5HT2A and 5HT2C receptors. The role of the GAD67 enzyme, which is involved in the synthesis of GABA, should also be mentioned here.
Earlier studies have shown that estrogen intake inhibits the expression of the GAD67 and 5HT2C receptor genes, suggesting that these neurons are involved in the control of ovulation.
The stress-sensitive monkeys showed increased expression of the 5HT2A receptor in the paraventricular nucleus of the hypothalamus, an area strongly involved in neurohormonal stress responses. They also had higher levels of the 5HT2C and GAD67 receptor in the pituitary stalk, an area of the brain important for ovulation. Higher GAD67 levels mean more GABA neurotransmitter, which in turn can inhibit gonadoliberin hormone (GnRH) and luteinizing hormone (LH) secretion. This in turn inhibits ovulation and reduces the secretion of estrogen and progesterone by the ovaries.
Corticoliberin (CRH)
Stressful situations activate the hypothalamic-pituitary-adrenal axis (HPA axis). The hypothalamus stimulates the pituitary gland, secreting the hormone corticoliberin (CRH). The pituitary gland under the influence of CRH secretes another hormone – corticotropin (ACTH). The corticotropin in turn is transported to the adrenal glands, which secretes the hormone cortisol.
Corticoliberin plays an important role in the stress response. It regulates behaviors such as activity, sleep, addiction tendency, and above all, anxiety-related behaviors. It also negatively affects fertility, inhibiting the secretion of luteinizing hormone. Previous studies have found that people with depression have four times more CRH neurons than healthy people. Bethea and her colleagues had similar results. Monkeys that experienced fertility problems rapidly as a result of stress had a greater number of CRH neurons in the paraventricular nucleus of the hypothalamus than stress-resistant monkeys. Increased secretion of corticoliberin in the raphe nucleus was also observed. Increased secretion of this hormone in this region may reduce serotonin secretion and lead to a reduction in fertility.
Proopiomelanocortin (POMC)
The neuropeptide beta-endorphin, which is formed from proopiomelanocortin (POMC), is also involved in the regulation of ovulation. Beta-endorphin-containing neurons inhibit the neuronal activity of gonadoliberin in the luteal phase of the menstrual cycle. Stress-sensitive animals are likely to suddenly activate POMC neurons and release beta-endorphins, another mechanism that reduces the ability to fertilize.
Summing up, it should be noted that the levels of these hormones and neurotransmitters were measured during the period when the animals were not subjected to stress. This may suggest that vulnerable and anxious people who are easily stressed have a reduced ability to fertilize, even if they are not stressed. On the other hand, living under stress increases these problems even more, so such people should especially take care of their well-being and try to relieve everyday stress. It is also worth noting that the experiment examined the impact of not only mental stress, but also the so-called metabolic stress (diet and exercise). Some people are more sensitive to these types of stressors, and a diet that is too low in calories to lose weight or engaging in sports too much can quickly make it difficult for them to become pregnant.
References
Bethea C., Centeno M. L. , Cameron J. (2008). Neurobiology of Stress-Induced Reproductive Dysfunction In Female Macaques. Molecular Neurobiology, 38(3), 199–230.
Author: Maja Kochanowska





Add comment